- There's one thing that all rubber, natural and synthetic, has in common. All rubber is stretchy. That's what makes it rubber, now isn't it? Just what makes rubber stretchy? The answer has to withentropy. What is entropy? Entropy is disorder. There is an important law of physics, called the Second Law of Thermodynamics which says that a system will move from a state of order to a state of disorder. We've all seen this in everyday life. A room is easy to make messy, but hard to make clean. It's easy to crash a new car, but hard to repair it afterward.
This entropy business is often inconvenient, but entropy is also what makes rubber work. Think about a piece of rubber. Remember that rubber molecules are polymers, that is they are shaped like very long chains. When the piece of rubber is just sitting there, the molecules are just tangled up in a random mess, like this:
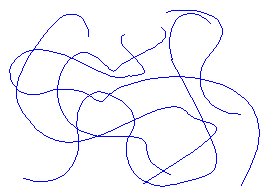

You can observe this happening. That's right you really can. One way to do this is the take a rubber band with your two hands and stretch it. While its stretched, hold it to your face. It feels hot doesn't it? Now release the tension on the rubber band so it can snap back to its original size. Now hold it to your face. How does it feel now? It should feel cold.
There's a reason for all this. When you pull on the rubber band, the polymer chains become aligned, remember? When the chains align, something can happen. The chains can line up and pack together into extremely ordered arrangements called crystals. This is how the rubber molecules are arranged in a crystal:
 rubber molecules in a crystal
rubber molecules in a crystalCrosslinking
There's something else that makes rubber stretchy. That is crosslinking. Most rubber objects are made of some kind of crosslinked rubber. Crosslinking is a way of chemically joining all the polymer chains of a piece of rubber into one giant molecule. Take a look at the picture. You can see the difference between a polymer that is crosslinked and one that isn't.
No comments:
Post a Comment